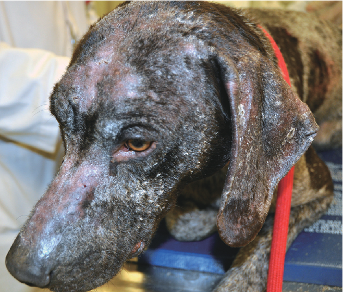
With the help of PennGen and their amazing Genetic Testing , Blue Line Gun Dogs has tested EVERY GSP we own for this terrible disease! Lupoid Dermatosis! Our Veterinarian visited our kennel and submitted blood from each dog for testing.
Every dog in our Breeding Program is CLEAR this terrible disease.
No puppy produced here at Blue Line Gun dogs will suffer from this Disease!
We want to do everything we can to better this breed and PREVENT Lupoid Dermatosis in our dogs and future puppies from our kennel. We love this breed!
We will NEVER stop testing for these terrible diseases that work to destroy
our beloved dogs.
our beloved dogs.
All Genetic test results are always public! Always Posted!
-Blue Line Gun Dogs
We use the OFA to test our dogs hips & elbows.
We use Wisdom Panel to test our dogs for Health & Genetics.
If you click the Picture above it will take you to their website and you can purchase your own testing for your personal dogs! We recommend this for any breed of dog! Or click here: WISDOM
Below you will find a lot of information on the Diseases we test our dogs for.
We go the extra mile to make sure we only produce Quality and truly healthy dogs/puppies.
Normal Veterinarian "Check-up's" will NOT determine if your dog has risk of these diseases!
Only Genetic Testing can.
Dogs may be a Carrier to one or more of the following diseases and without testing you cannot know.
If a Carrier Dog/Bitch is accidentally bred to another Carrier you then have Affected puppies. (Puppies who will SUFFER)
Many ask us why we test.
Our response to them is, WHY NOT!
We want to preserve the GSP Breed. We take great pride in our dogs and breeding program we have developed.
Blue Line Gun Dogs will continue to TEST and when new testing becomes available we will have it done.
The preservation of this breed depends on it.

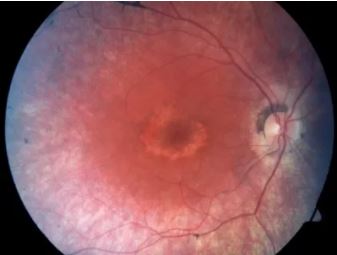
Cone degeneration (German Shorthaired Pointer type) is an inherited eye disease affecting dogs. Affected dogs develop day-blindness (blindness in bright light) and Photophobia (light sensitivity) between 8 to 12 weeks after birth due to degeneration of cells in the eye called cone photoreceptors which are responsible for vision in bright light. Affected dogs have normal vision in low light and structures of the inner eye appear normal on a veterinary eye exam. Normal cone cell function can be seen on Electroretinogram (ERG) before six weeks of age, but becomes abnormal between 6 to 12 weeks of age and is completely absent in affected adult dogs signifying complete loss of Cone Cells. The cells responsible for vision in low light called Rod photoreceptors are not affected and thus, affected dogs will still be able to see normally in low light throughout life.
Testing Tips:
Genetic testing of the CNGB3 gene will reliably determine whether a dog is a genetic Carrier of cone degeneration (German
shorthaired pointer type). Cone degeneration (German shorthaired pointer type) is inherited in an Autosomal
Recessive manner in dogs meaning that they must receive two copies of the mutated gene (one from each parent) to
develop the disease. In general, carrier dogs do not have features of the disease but when bred with another carrier of the
same Mutation, there is a risk of having affected pups. Each pup that is born to this pairing has a 25% chance of inheriting
the disease and a 50% chance of inheriting being a carrier of the CNGB3 gene. Reliable genetic testing is important for
determining breeding practices. In order to eliminate this mutation from breeding lines and to avoid the potential of
producing affected pups, breeding of known carriers to each other is not recommended. Dogs that are not carriers of the
mutation have no increased risk of having affected pups.


Von Willebrand disease type II (VWDII) is an inherited bleeding disorder affecting
dogs. Dogs affected with VWDII have decreased levels and abnormal function of von
Willebrand coagulation factor (vWf), which is an essential protein needed for normal
blood clotting. Affected dogs generally have moderate to severe signs of a bleeding
disorder. Affected dogs may bruise easily, have frequent nosebleeds, bleed from the
mouth when juvenile teeth are lost and experience prolonged bleeding after surgery or
trauma. The bleeding may be severe enough to cause death. Due to variable severity of
the disorder, affected dogs may not be identified until a surgery is performed or trauma
occurs at which time excessive bleeding is noted. Veterinarians performing surgery on
known affected dogs should have ready access to blood banked for transfusions. Dogs
can have a normal lifespan with this condition although they are susceptible to life-
threatening bleeding with an accidental injury or any surgical procedure.\
Testing Tips
Genetic testing of the VWF gene will reliably determine whether a dog is a genetic Carrier of von Willebrand disease II. Von Willebrand disease II is inherited in an Autosoma Recessive manner in dogs meaning that they must receive two copies of the mutated gene (one from each parent) to develop the disease. In general, carrier dogs do not have features of the disease but when bred with another carrier of the same Mutation, there is a risk of having affected pups. Each pup that is born to this pairing has a 25% chance of inheriting the disease and a 50% chance of being a carrier of the VWF gene mutation. Reliable genetic testing is important for determining breeding practices. In order to eliminate this mutation from breeding lines and to avoid the potential of producing affected pups, breeding of known carriers to each other is not recommended. Dogs that are not carriers of the mutation have no increased risk of having affected pups.
Refrences:
www.pawprintgenetics.com
www.facebook.com (German Shorthaired Pointer Group) (Picture)
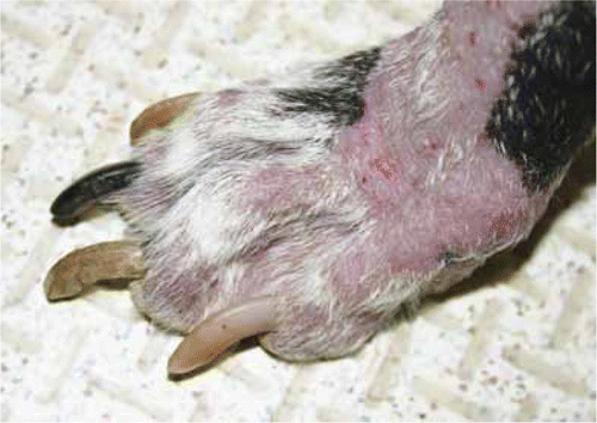
Acral Mutilation Syndrome
Pain Insensitivity, Sensory Neuropathy, AMS, SN
Common Symptoms
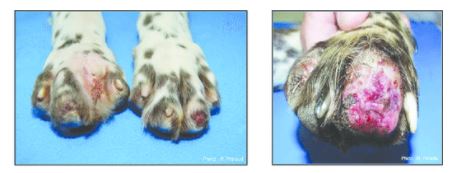
Acral mutilation syndrome is an inherited neurological disease affecting dogs. Affected dogs present around 4 months of age with an insensitivity to pain in the lower limbs and repetitive licking and biting of their paws, eventually resulting in self-mutilation. Affected dogs are often smaller than littermates and present with severe, self-induced wounds of the feet including toe amputations, loss of toenails, and bone fractures. Dogs with open wounds are subject to additional complications associated with secondary infections. Since affected dogs are unable to feel pain in their feet, they will continue to walk without obvious discomfort. Euthanasia is often requested by owners due to quality of life concerns and a lack of treatment options.
Testing Tips
Genetic testing of the GDNF gene will reliably determine whether a dog is a genetic Carrier of acral mutilation syndrome. Acral mutilation syndrome is inherited in an Autosomal Recessive manner in dogs meaning that they must receive two copies of the mutated gene (one from each parent) to develop the disease. In general, carrier dogs do not have features of the disease but when bred with another carrier of the same Mutation, there is a risk of having affected pups. Each pup that is born to this pairing has a 25% chance of inheriting the disease and a 50% chance of inheriting one copy and being a carrier of the GDNF gene mutation. Reliable genetic testing is important for determining breeding practices. In order to eliminate this mutation from breeding lines and to avoid the potential of producing affected pups, breeding of known carriers to each other is not recommended. Dogs that are not carriers of the mutation have no increased risk of having affected pups.
There may be other causes of this condition in dogs and a normal result does not exclude a different mutation in this gene or any other gene that may result in a similar genetic disease or trait.
References: www.pawprintgenetics.com
- Cummingss JF, de Lahunta A, Winn SS. Acral mutilation and nociceptive loss in English pointer dogs. A canine sensory neuropathy. Acta Neuropathol. 1981;53(2):119-27. [PubMed: 6259871]
- Plassais J, Lagoutte L, Correard S, Paradis M, Guaguere E, Hedan B, Pommier A, Botherel N, Cadiergues M, Pilorge P, Silversides D, Bizot M, Samuels M, Arnan C, Johnson R, Hitte C, Salbert G, Mereau A, Quignon P, Derrien T, Andre C. A Point Mutation in a lincRNA Upstream of GDNF Is Associated to a Canine Insensitivity to Pain: A Spontaneous Model for Human Sensory Neuropathies. PLoS Genet. 2016 Dec 29;12(12):e1006482. [PubMed: 28033318]

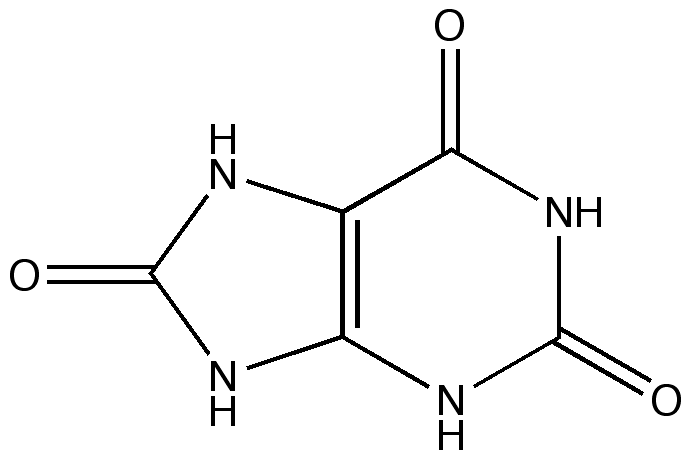
HYPERURICOSURIA
Common Symptoms
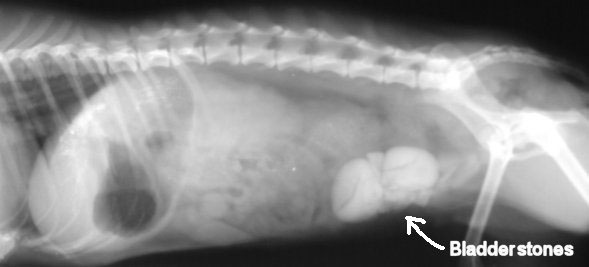
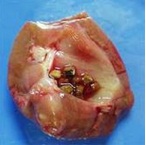
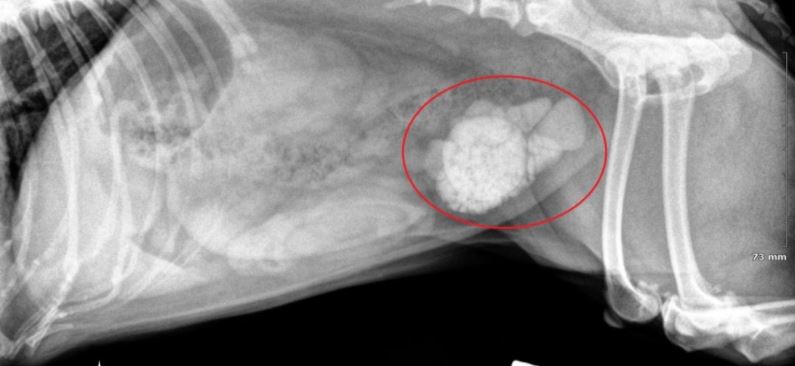
Hyperuricosuria is an inherited condition of the urinary system affecting several breeds of dog. The SLC2A9 gene codes for a protein that allows the kidneys to transport uric acid from the urine. Dogs with mutations in both copies of the SLC2A9 gene are predisposed to have elevated levels of uric acid in the urine, hence the name hyperuricosuria. Uric acid can form crystals and/or stones (uroliths) in the urinary tract. Dogs with hyperuricosuria most commonly present with symptoms of recurrent urinary tract inflammation, which include frequent urination, blood in the urine, and straining to urinate. They may also have loss of appetite, lethargy, weakness, vomiting and pain. Urinary stones in the bladder can cause urinary tract infections or more seriously, blockage of the Urethra. Both male and female dogs can be affected, but obstruction of urine flow is more common in males due to differences in anatomy. Although an x-ray can be used to exclude other types of stones, urate stones cannot typically be seen using x-rays and must be evaluated by ultrasound. Not all dogs with mutations in both copies of the SLC2A9 gene will have symptoms of disease, though they will have increased uric acid excretion in the urine.
Breed-Specific Information for the German Shorthaired Pointer
The Mutation of the SLC2A9 gene associated with hyperuricosuria has been identified in the German shorthaired pointer, although its overall frequency in this breed is unknown.
Testing Tips
Genetic testing of the SLC2A9 gene in German shorthaired pointer will reliably determine whether a dog is a genetic Carrier of hyperuricosuria. Hyperuricosuria is inherited in an Autosomal Recessive manner in dogs meaning that they must receive two copies of the mutated gene (one from each parent) to develop the disease. In general, carrier dogs do not have features of the disease but when bred with another carrier of the same Mutation, there is a risk of having affected pups. Each pup that is born to this pairing has a 25% chance of inheriting the disease and a 50% chance of inheriting one copy and being a carrier of the SLC2A9 gene mutation. Reliable genetic testing is important for determining breeding practices. Because not all affected dogs will have clinical signs associated with hyperuricosuria, genetic testing should be performed before breeding. In order to eliminate this mutation from breeding lines and to avoid the potential of producing affected pups, breeding of known carriers to each other is not recommended. German shorthaired pointers that are not carriers of the mutation have no increased risk of having affected pups.
Refrences: www.pawprintgenetics.com
- Bannasch D, Safra N, Young A, Karmi N, Schaible RS, Ling GV. Mutations in the SLC2A9 gene cause hyperuricosuria and hyperuricemia in the dog. PLoS Genet. 2008 Nov;4(11):e1000246. [PubMed: 18989453]
- Cosgrove L, Hammond G, Mclauchlan G. PRIMARY portal vein hypoplasia AND SLC2A9 mutation associated WITH urate urolithiasis IN a Spanish water dog. Can Vet J. 2015 Nov;56(11):1153-7. [PubMed: 26538670]
- Donner J, Kaukonen M, Anderson H, Moller F, Kyostila K, Sankari S, Hytonen M, Giger U, Lohi H. Genetic Panel Screening of Nearly 100 Mutations Reveals New Insights into the Breed Distribution of Risk Variants for Canine Hereditary Disorders. PLoS One. 2016 Aug 15;11(8):e0161005. [PubMed: 27525650]
- Karmi N, Brown EA, Hughes SS, McLaughlin B, Mellersh CS, Biourge V, Bannasch DL. Estimated frequency of the canine hyperuricosuria mutation in different dog breeds. J Vet Intern Med. 2010 Nov-Dec; 24(6):1337-42. [PubMed: 21054540]
- Karmi N, Safra N, Young A, Bannasch DL. Validation of a urine test and characterization of the putative genetic mutation for hyperuricosuria in Bulldogs and Black Russian Terriers. Am J Vet Res. 2010 Aug; 71(8):909-14. [PubMed: 20673090]